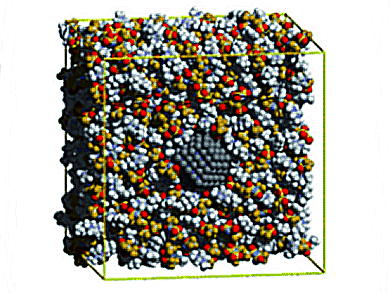
Some room-temperature ionic liquids can hold stable suspensions of nanoparticles without additional surface-active agents through mechanisms of solvation and stabilization. The mechanisms are not understood at present, particularly for metallic nanoparticles. These systems are relevant for applications in catalysis, lubrication, electrochemical devices, and chemical processes.
Alfonso S. Pensado and Agilio A. H. Padua, Universite Blaise Pascal Clermont-Ferrand & CNRST, Aubiere, France, investigated the solvation of a metallic nanoparticle in the ionic liquid [C4C1im][Ntf2] by molecular simulation with a specific interaction potential. The interfacial layer of ionic liquid is only one ion thick, and thus excludes a stabilization mechanism
based on an electrostatic double layer. The stabilization mechanism is also not likely to be due to steric effects of the alkyl chains in the cations, which are too short in the present case.
A stabilization effect of the solvent as structural matrix or template is assumed, as the length scales of the ionic liquid affect the nucleation and growth of the nanoparticles, and also the stability of the resulting colloid.
- Solvation and Stabilization of Metallic Nanoparticles in Ionic Liquids,
Alfonso S. Pensado, Agilio A. H. Padua,
Angew. Chem. Int. Ed. 2011, 50.
DOI: 10.1002/anie.201103096