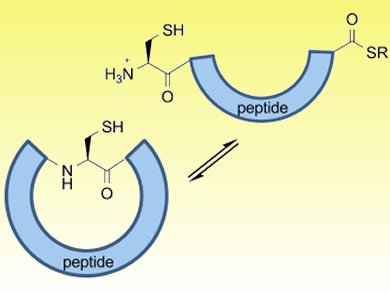
Derek Macmillan and colleagues, University College London, UK, report a simple new route to cyclic peptides from unactivated linear precursors. In the presence of a thiol, an N→S acyl shift in linear peptides can give thioesters, key components for native chemical ligation (NCL). These transient C-terminal thioesters can be intercepted by an N-terminal cysteine to form a new amide bond through NCL and result in biologically active cyclic products.
The products are of considerable interest because peptide cyclization is known to increase the therapeutic potential of many peptides by increasing their thermal and proteolytic stability as well as oral bioavailability.
Image: © Wiley-VCH
- Synthesis of Cyclic Peptides through an Intramolecular Amide Bond Rearrangement
D. Macmillan, M. De Cecco, N. L. Reynolds, L. F. A. Santos, P. E. Barran, J. R. Dorin,
ChemBioChem 2011.
DOI: 10.1002/cbic.201100364