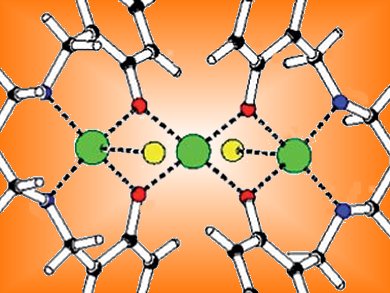
The halogenation of aromatic rings under mild conditions is hard to perform and the synthetic methods currently available are not very selective.
Jan Reedijk and co-workers, Leiden University, the Netherlands, have performed an oxidative halogenation which occurs with high selectivity for the 5-position on the aromatic ring. The reaction of bis(2-hydroxybenzyl)-1,3-diaminopropane (H2bhbd) with CuCl2 in acetonitrile generated linear trinuclear [Cu3(bhcbd)2Cl2](CH3CN) (see picture). The ligands of this cluster contained phenol moieties which had been chlorinated. Bromination could also be achieved by using CuBr2 instead of CuCl2.
The reactions can be performed under exceptionally mild conditions, i.e., at room temperature, using air as the sole oxidant, and with cheap and non-toxic CuX2 as the halide source.
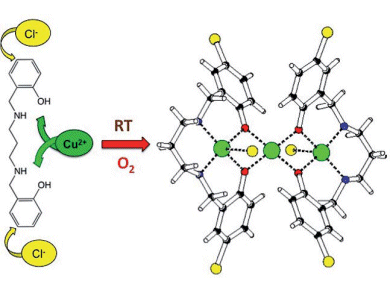
Image: © Wiley-VCH
- Selective Copper-Mediated Halogenation of Aromatic Rings Under Mild Conditions
I. Gamba, P. Gamez, E. Monzani, L. Casella, I. Mutikainen, J. Reedijk,
Eur. J. Inorg. Chem. 2011.
DOI: 10.1002/ejic.201100489