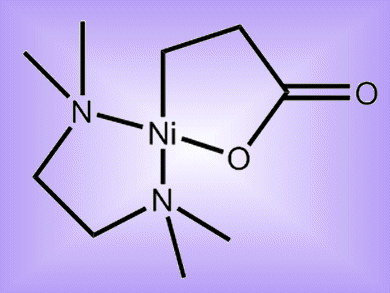
The prospect of using carbon dioxide to produce value-added products is very attractive for the chemical industry. Nickelalactones are formed from the reaction of C2H4 and CO2 at Ni0 centers. Treatment of these with methyl iodide results in the formation of an alkyl ester ligand, leading to the release of methyl acrylate through β-hydride elimination. This reaction is so far not catalytic and the yields are rather low (max. 33 %).
Fritz Kühn and colleagues, Technical University Munich, Germany, have shown that nickelalactones bearing chelating diamines or diphosphines can produce methyl acrylate in yields of up to 56 %.
The efficiency of the reaction was found to depend on the strength of the nickel–ligand bond. A loosely bound ligand could dissociate one arm to reduce steric hindrance and facilitate approach of the β-H atom towards the nickel center. The efficiency of the reductive elimination of methyl acrylate was dependent on the ligand due to electronic and steric effects on the nickel atom.
- Transformation of Nickelalactones to Methyl Acrylate: On the Way to a Catalytic Conversion of Carbon Dioxide
S. Y. T. Lee, M. Cokoja, M. Drees, Y. Li, J. Mink, W. A. Herrmann, F. E. Kühn,
ChemSusChem 2011.
DOI: 10.1002/cssc.201000445