Splitting bonds is the key step in most catalytic chemical transformations. For many years, it has been performed quasi-exclusively by transition-metal complexes; it is of paramount importance to find alternatives that are less costly and more environmentally friendly.
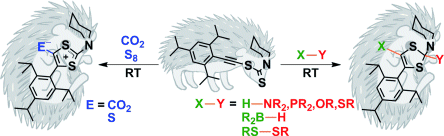
Guy Bertrand and co-workers, University of California, Riverside, USA, have shown that the simple and apparently benign molecule, ethynyl dithiocarbamate [Ar—C≡C—S—C(S)NR2], is able to cleave a broad range of strong σ bonds even at room temperature. Bond activation involves either equilibrium with the non-observable cyclic mesoionic carbene isomer or the cooperation of the nucleophilic carbon–carbon triple bond and the electrophilic CS carbon atom. Both routes lead to cyclic products.
Analogues of ethynyl dithiocarbamate featuring other heteroatoms are readily available, allowing a fine-tuning of the electronic properties of this type of bond activators and opening the way for organocatalytic substrate activation.
Images: © Wiley-VCH
- Bond Activation with an Apparently Benign Ethynyl Dithiocarbamate Ar—C≡C—S—C(S)NR2
G. Ung, G. D. Frey, W. W. Schoeller, G. Bertrand,
Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201104303