Direct conversion of an aryl C–H bond into an aryl–aryl bond alleviates the need for prefunctionalization — halogenation and/or metalation — of the aromatic substrates. Reactions reported so far typically rely on the use of expensive transition metal catalysts and require harsh reaction conditions.
Eiichi Nakamura and colleagues, University of Tokyo, Japan, perform the direct arylation of the ortho-C–H bond of an aryl pyridine or an aryl imine with an aryl Grignard reagent. The method employs an iron-diamine catalyst and a dichloroalkane as oxidant at 0 °C. The key to the reaction is the presence of an aromatic co-solvent, such as chlorobenzene and benzene, and the slow addition of the Grignard reagent.
Aryl–aryl bond formation takes place in 5 minutes, substantially faster than reactions catalyzed by precious metals at elevated temperatures.
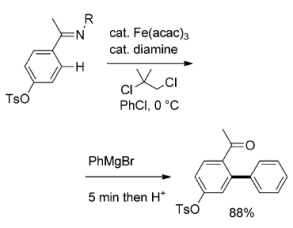
- Iron-Catalyzed C–H Bond Activation for the ortho-Arylation of Aryl Pyridines and Imines with Grignard Reagents
N. Yoshikai, S. Asako, T. Yamakawa, L. Ilies, E. Nakamura,
Chem. Asian J. 2011.
DOI: 10.1002/asia.201100470