Color and Decomposition
Autumn is right around the corner in the northern hemisphere and the leaves are beginning to change color. The cause of this wonderful display of reds, yellows, and oranges is the decomposition of the compound that makes leaves green: chlorophyll. Bernhard Kräutler and a team at the University of Innsbruck, Austria, have now published a report in the journal Angewandte Chemie about the discovery of a previously unknown chlorophyll decomposition product in the leaves of Norway maples. The different spatial arrangement of its atoms is indicative of a different decomposition pathway than those of other deciduous trees.
During the summer months, green leaves carry out photosynthesis: chlorophyll converts sunlight into chemical energy. In the fall, deciduous trees reabsorb critical nutrients, such as nitrogen and minerals, from their leaves. This releases the chlorophyll from the proteins that normally bind it. However, chlorophyll is phototoxic in this free from, and can damage the tree when exposed to light. It must therefore be “detoxified” by decomposition.
“Essential pieces of the puzzle of this biological phenomenon have been solved only within the last two decades,” reports Kräutler. Various colorless tetrapyrroles, molecules with a framework of four nitrogen-containing five-membered carbon rings, accumulate in the dying leaves of higher plants, and have been classified as decomposition products of chlorophyll. These are called “nonfluorescent” chlorophyll catabolytes (NCCs). Says Kräutler, “ they are considered to be the final breakdown products of a well-controlled, “linear” and widely common decomposition pathway.” This premise is beginning to get a little shaky.
Different Chlorophyll Breakdown Pathway
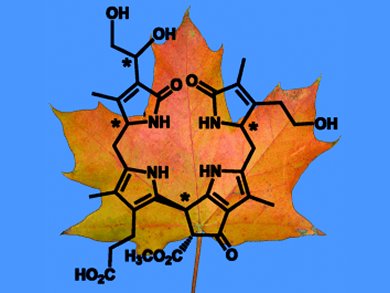
Kräutler and his co-workers have studied the decomposition of chlorophyll in the Norway maple, a tree native to Eurasia. “We found none of the typical breakdown products in yellow-green or yellow Norway maple leaves,” says Kräuter. “Instead, the main product we found was a dioxobilane, which resembles a chlorophyll breakdown product found in barley leaves.”
However, there are small but important differences in the spatial arrangements of the atoms relative to each other. There is no plausible decomposition pathway that starts with the NCCs and leads to this new decomposition product. “There is clearly a chlorophyll breakdown pathway occurring in Norway maple leaves that differs from those previously known.”
The structure of this newly discovered dioxobilane is reminiscent of bile pigments, which are products of the breakdown of heme, and thus are important constituents of mammalian metabolisms as well as acting as light sensors in plants. “This supports the idea that chlorophyll breakdown is not only a detoxification process; the resulting decomposition products can also play a physiological role,” states Kräuter. “Chlorophyll breakdown products can act as antioxidants in the peel of ripening fruits, making the fruits less perishable. What role they play in leaves is not yet clear.”
- A Dioxobilane as Product of a Divergent Path of Chlorophyll Breakdown in Norway Maple

T. Müller, M. Rafelsberger, C. Vergeiner, B. Kräutler,
Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201103934