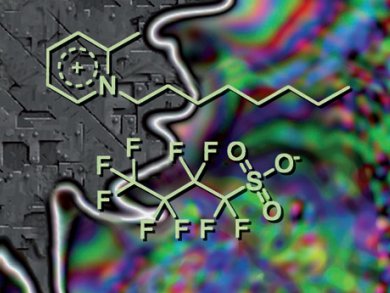
Organic salts are ubiquitous in pharmaceuticals, pigments, detergents, electrolytes, explosives, catalysts, reactants in chemical syntheses and many other applications. Ionic liquids (ILs) are defined as those organic salts that have melting points below 100 °C. It has been estimated that there are 106 to 1018 possible ion combinations that will yield ILs. Computer-aided synthesis and high-throughput screening of substances could identify ways to fine-tune an IL structure before its first synthesis and allow the assembly of suitable candidate ILs from a database of single ions. For this, a reliable procedure to predict the melting point of an IL is needed.
Ingo Krossing and co-workers, Freiberg University, Germany, have developed an automated thermodynamic melting point prediction protocol. They evaluated the quality and reproducibility of experimentally determined melting point data and assigned a rough error bar of ±5 to ±15 °C. With this restraint, the team developed two simple, semiempirical, five- and nine-parameter schemes with easy-to-calculate quantities. These were validated for 520 salts with a relative error of 9.3 %. The schemes can predict the melting temperature for a large variety of ionic compounds, from acyclic to (homo- or hetero-)polycyclic, in the temperature range of –25 to +300 °C.
This could help design new ILs for a given application in a certain temperature range.
- Is Universal, Simple Melting Point Prediction Possible?
U. P. Preiss, W. Beichel, A. M. T. Erle, Y. U. Paulechka, I. Krossing,
ChemPhysChem 2011.
DOI: 10.1002/cphc.201100522