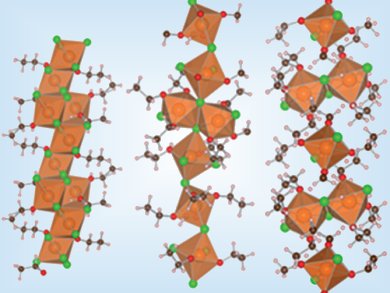
Ziegler–Natta polymerization is used to produce polyolefins on a multimillion-ton scale worldwide. The production of supported catalysts for this reaction starts with magnesium chloride modified by Lewis bases, typically ethanol. The productivity of the catalysts and the isotacticity of the polymers strongly depend on the Lewis base-to-MgCl2 ratio. Control over the structure of MgCl2•nEtOH adducts is crucial to understanding and tailoring catalyst activity.
Giuseppe Cruciani and colleagues, University of Ferrara, Italy, report the first crystal-structure determination for three MgCl2•nEtOH complexes, namely, 2MgCl2•3EtOH (n=1.5), 5MgCl2•14EtOH (n=2.8) and 3MgCl2•10EtOH (n=3.3) (pictured, left to right). The structures of MgCl2•nEtOH complexes with n=1.5 and 2.8 are based on ribbons of metal-centered octahedra. For n=3.3, this chainlike arrangement breaks into a threadlike structure of isolated octahedra linked by hydrogen bonds.
Knowledge of these three structural models allows a complete atomic-scale structural description of the majority of Ziegler–Natta catalyst supports. This will help develop more accurate models for activated catalysts that avoid the oversimplification of current models and could lead to the targeted design of Ziegler–Natta catalysts.
Image: © Wiley-VCH
- Crystal Structures of Ziegler–Natta Catalyst Supports
F. Malizia, A. Fait, G. Cruciani,
Chem. Eur. J. 2011.
DOI: 10.1002/chem.201101900