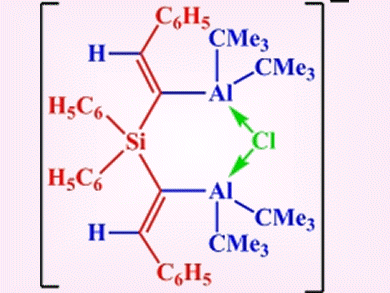
Chelating Lewis acids with a geminal arrangement of two acceptor functions have been shown to effectively coordinate halide, thiolate, or benzoate anions. However, the acceptor atoms usually occupy geminal positions at a bridging carbon atom, which results in relatively strained four-membered heterocycles upon coordination of single-atom donors.
Werner Uhl and colleagues, University of Münster, Germany, employed the twofold hydroalumination of silicon-centered dialkynes to prepare compounds that have larger spacers between the acceptor functions. This allows more flexible backbones and hence better coordinating properties. A compound prepared in this way was shown to chelate chloride ions to give a six-membered ClAl2C2Si heterocycle (pictured).
- Hydroalumination of Bis(alkynyl)silanes: Generation of Chelating Lewis Acids, Their Application in the Coordination of Chloride Ions and a 1,1-Carbalumination Reaction
W. Uhl, D. Heller, J. Kösters, E.-U. Würthwein, N. Ghavtadze,
Eur. J. Inorg. Chem. 2011.
DOI: 10.1002/ejic.201100890