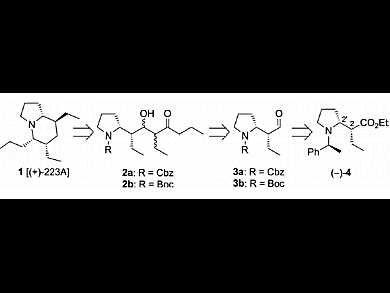
Indolizidine and quinolizidine ring systems constitute the core of numerous structurally interesting alkaloids extracted from the skins of amphibians. The small amounts isolated and the potent biological activities of these natural products make them attractive targets in organic synthesis. To date, five syntheses of indolizidine 223A have been reported. Most of them involve the elaboration of a chiral tetrasubstituted piperidine intermediate and its subsequent cyclization into the corresponding indolizidine.
Mouloud Fellah, Gérard Lhommet, and Virginie Mouriès-Mansuy, UPMC University of Paris, Sorbonne Universités, France, report an efficient total formal synthesis of indolizidine (+)-223A, the opposite enantiomer of natural (–)-223A. Their strategy was based on three major steps: chain elongation by aldolization, formation of a bicyclic enone by cyclization, and stereocontrolled hydrogenation of the obtained tetrahydroindolizinone. Expected indolizidine (+)-223A was obtained in 10 % yield over 14 steps starting from readily available ethyl 6-chlorohex-2-ynoate.
Application of this flexible route to the synthesis of more complex alkaloids is currently under investigation.
- Total Synthesis of Indolizidine (+)-223A,
Mouloud Fellah, Gérard Lhommet, Virginie Mouriès-Mansuy,
Eur. J. Org. Chem. 2011.
DOI: 10.1002/ejoc.201101530