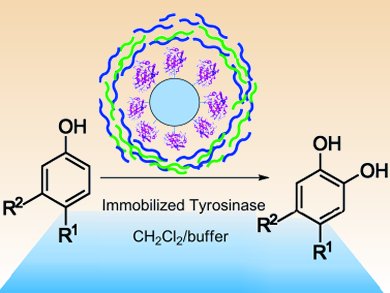
Catechols are a family of bioactive substances that can be derived from phenols. Their environmentally friendly synthesis is, however, a sticking point in biochemistry. Raffaele Saladino and co-workers, University of Tuscia, Italy, have shown how mushroom tyrosinase, immobilized on a solid support, can catalyze the oxidation of phenols to their corresponding catechols.
After immobilization on Eupergit C 250 L, the mushroom tyrosinase was stabilized further by a coating of polyelectrolyte layers, formed by means of the layer-by-layer technique. The enzyme was suspended first in positively, then in negatively charged electrolyte solutions. This process was repeated twice to build up a three-layer coating around the enzyme. Although slightly decreasing the activity, the polyelectrolyte layers were found to enhance the selectivity and stabilization of the tyrosinase.
The immobilized tyrosinase selectively oxidized para– and meta-substituted phenols; ortho-derivatives were not oxidized. Furthermore, kinetic investigations showed the enzyme to be more reactive in organic than buffer solution. The system, which works at room temperature, retained catalytic activity over successive runs and provides a mild and environmentally friendly route to catechols.
Image: © Wiley-VCH
- A Novel Synthesis of Bioactive Catechols by Layer-by-Layer Immobilized Tyrosinase in an Organic Solvent Medium,
M. Guazzaroni, M. Pasqualini, G. Botta, R. Saladino,
ChemCatChem 2012, 4, 89–99.
DOI: 10.1002/cctc.201100229