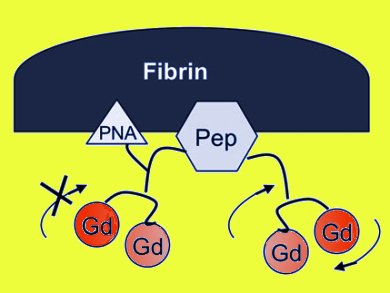
Magnetic resonance imaging (MRI) is a popular non-invasive technique that enables the visualization of changes in tissues, a great help in the diagnosis of many diseases. Paramagnetic gadolinium(III) complexes are widely used to increase contrast. Relaxivity is a measure of the ability of magnetic compounds to increase the relaxation rates of the proton spins of the surrounding water molecules. This is an inherent property of imaging agents affecting the contrast, and hence the quality of the image. Relaxivity depends on the structure of the complex, the kinetics of inner-sphere and second-sphere water exchange, and the rotational dynamics of the molecule. However, these properties change when the contrast agent is bound to its target.
Peter Caravan and Zhaoda Zhang, A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital and Harvard Medical School, USA, summarize the efforts in their group to understand and optimize the relaxivity of contrast agents targeted to serum albumin and to fibrin (pictured). They conclude that inner-sphere water-exchange rates can be controlled by judicious use of donor groups and that the second-hydration sphere can also be tuned to augment relaxivity further. Control of rotational dynamics is a particularly powerful lever for increasing relaxivity.
The dual binder strategies described for serum albumin- and fibrin-targeted agents are generalizable to other targeted contrast agents.
Image: © Wiley-VCH
- Structure–Relaxivity Relationships among Targeted MR Contrast Agents,
Peter Caravan, Zhaoda Zhang,
Eur. J. Inorg. Chem. 2012.
DOI: 10.1002/ejic.201101364