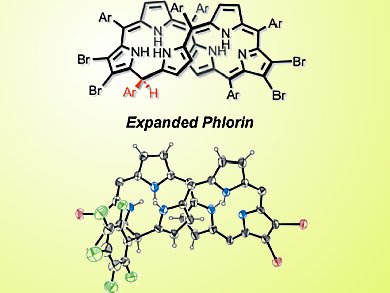
Phlorins are expanded porphyrins that feature a saturated sp3 meso carbon atom and thus do not have the typical 18-π-electron cyclic conjugation of other porphyrinoids. This altered conjugation gives rise to very different optical properties. But the phlorins are also instrinsically unstable and thus difficult to investigate.
Atsuhiro Osuka and Tomohiro Higashino at Kyoto University, Japan, report the synthesis of isolable phlorin-type hexaphyrins.
Condensation of 3,4-dihalopyrroles and a dipyrromethane-dicarbinol under acid catalysis and subsequent oxidation yields 2,3,17,18-tetrahalogeno [26]- and [28]hexaphyrins. NMR spectra reveal equilibria of different conformers in solution. The [28]hexaphyrins are then reduced with a large excess of sodium borohydride to give the first examples of phlorin-type hexaphyrins.
- 2,3,17,18-Tetrahalohexaphyrins and the First Phlorin-type Hexaphyrins,
Tomohiro Higashino, Atsuhiro Osuka,
Chem. Asian J. 2013, 8.
DOI: 10.1002/asia.201300474