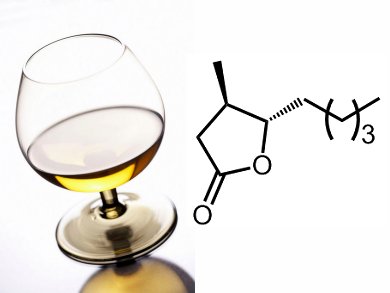
Volatile γ-lactones are popular components of flavorings and fragrances, but their synthesis from O-allyl-α-haloesters by radical cyclization is hindered by slow conformational equilibration.
Fabrice Dénès and co-workers, Université de Nantes, France, have shown that α-bromo aluminum acetals, prepared from the corresponding α-bromo esters using diisobutylaluminum hydride (also known as DIBAL-H) can be cyclized at low temperature under reducing conditions. They report that this method can be combined in a one-pot sequence with subsequent Oppenauer oxidation to prepare the desired γ-lactones. This process enables straightforward access to γ-lactones from easily available precursors. The efficiency of the methodology was illustrated by synthesis of optically enriched (–)-trans-cognac lactone (pictured).
- A Convenient Access to γ-Lactones from O-Allyl-α-Bromoesters using a One-Pot Ionic–Radical–Ionic Sequence,
Romain Bénéteau, Jacques Lebreton, Fabrice Dénès,
Chem. Asian J. 2012.
DOI: 10.1002/asia.201200212