A mild, efficient and widely-applicable method for incorporating alkyne fragments into complex molecules, including steroids and substituted benzofuranes, is described by José Luis García Ruano, José Alemán, and colleagues, Universidad Autónoma de Madrid, Spain.
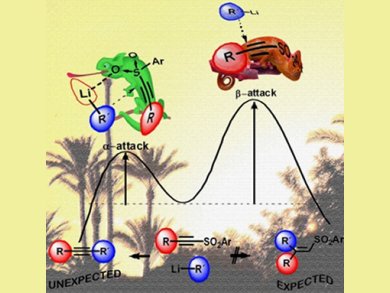
The authors have explored a 1979 report by William Truce and Roland Smorada, Purdue University, USA, describing the synthesis of aryl-alkynes from the reaction of organolithiums and arylsulfonylacetylenes, which was until now generally considered a scientific curiosity. In fact, this reaction presents a potential alternative to Sonogashira or other specialized metal-catalyzed reactions, as it requires less expensive reagents, generates less waste, and is applicable to nearly all Csp2–Csp and Csp3–Csp bonds. It is, thus, an important discovery for the areas of bioactive natural product synthesis, new materials, and biochemistry.
Theoretical calculations by Garcia Ruano’s team suggest that the chameleonic behavior of the sulfonyl group, which coordinates to the organolithium, allows an α- rather than β-attack of the nucleophile in an anti-Michael addition of the alkyne fragment to the original substrate.
Image: © Wiley-VCH
- Expanding the Scope of Arylsulfonylacetylenes as Alkynylating Reagents and Mechanistic Insights in the Formation of Csp2–Csp, Csp3–Csp Bonds from Organolithiums,
J. L. García Ruano, J. Alemán, L. Marzo, C. Alvarado, M. Tortosa, S. Díaz-Tendero, A. Fraile,
Chem. Eur. J. 2012.
DOI: 10.1002/chem.201200939
- Reactions of arylsulfonylacetylenes with organolithium and Grignard reagents: a new synthesis of acetylenes,
W. E. Truce, R. L. Smorada,
J. Org. Chem. 1979, 44, 3444.
DOI: 10.1021/jo01333a049