Aromatic compounds bearing one or more CF3 groups on the ring are important intermediates and building blocks for the synthesis of numerous modern pharmaceuticals, highly efficient crop-protection agents, and specialty materials.
Trifluoromethylated aromatic compounds are currently manufactured by exhaustive chlorination of a methyl group on the aromatic ring, followed by the Swarts reaction of the resultant ArCCl3 with HF. This process shows low functional-group tolerance and is ecologically unfriendly as it involves aggressive and hazardous materials (Cl2, HF) and generates large quantities of chlorine waste (HCl).
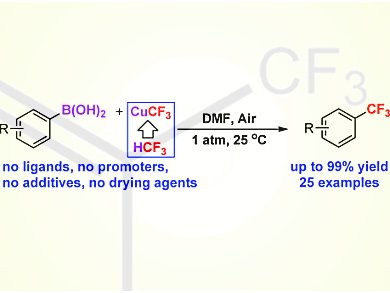
Vladimir V. Grushin and colleagues, Institute of Chemical Researcxh of catalonia (ICIQ), Tarragona, Spain, have demonstrated, for the first time, that low-cost fluoroform-derived CuCF3 reagents readily trifluoromethylate aryl boronic acids. The reaction smoothly occurs at room temperature and even below and 1 atm of air as the oxidant to give the corresponding benzotrifluorides in excellent yield (up to 99 %) and with high selectivity. The method exhibits unprecedentedly high functional-group tolerance for a variety of substrates bearing substituents in the ortho, meta, and para positions; even aryl boronic acids with formyl groups on the ring have been successfully .jpg) trifluoromethylated in 74–82 % yield.
trifluoromethylated in 74–82 % yield.
The use of additional ligands, costly oxidants (AgI), drying agents, and pure O2 is not needed, which makes the reaction not only synthetically useful and inexpensive, but also advantageously simple and safe to run.
- Fluoroform-Derived CuCF3 for Low-Cost, Simple, Efficient, and Safe Trifluoromethylation of Aryl Boronic Acids in Air,
Petr Novák, Anton Lishchynskyi, Vladimir V. Grushin,
Angew. Chem. Int. Ed. 2012, 51, 7767–7770.
DOI: 10.1002/anie.201201613