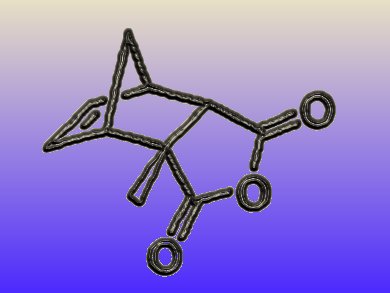
Five-membered lactones are natural products that are often produced by microorganisms. Depending on the exact structure, they possess diverse biological functions, ranging from multiple flavor properties to neurobiological effects. If the lactones are chiral, only one of the enantiomers might be responsible for the biological function. Therefore, an enantioselective synthesis of lactones is of great interest.
Jeroen Dickschat and colleagues, University of Braunschweig, Germany, found a new synthetic route towards chiral, substituted lactones with a Diels-Alder/retro-Diels-Alder approach. The scientists prepared an enantiomeric Diels-Alder adduct by using a chiral Lewis acid during the Diels-Alder reaction. As a key step, they carried out a diastereoselective aldoladdition on this Diels-Alder adduct. This allowed them to introduce a second stereocenter. Reversing the effects of the first Diels-Alder reaction with a retro-Diels-Alder reaction removed one stereocenter, leaving behind an enantiomer. Therefore, the researchers accomplished to isolate an almost enantiomerically pure lactone from a diastereoselective key reaction.
- A Diels–Alder/Retro-Diels–Alder Approach for the Enantioselective Synthesis of Microbial Butenolides,
C. A. Citron, S. M. Wickel, B. Schulz, S. Draeger, J. S. Dickschat,
Eur. J. Org. Chem. 2012.
DOI: 10.1002/ejoc.201200991