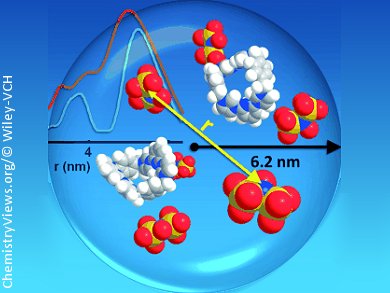
A central question in any application of solution-based ionic self-assembly is: Does any kind of ordered arrangement of the ionic building blocks take place? Intuitively one would assume the distribution of ions in solution to be of random, rather homogeneous nature.
Dariush Hinderberger and co-workers, Max Planck Institute for Polymer Research, Mainz, Germany, have observed long-ranged electrostatic correlations in a solution of low-molecular electrolytes. Using computational methods, including molecular dynamics (MD) and Monte Carlo (MC) simulations, and experimental data from dynamic light scattering (DLS), and double electron–electron resonance (DEER), they show that their ions are not distributed randomly in solution but are well-ordered.
These electrostatically self-assembled supramolecular structures (ionoids) are of well-defined size, have mobile interiors, and molecules within populate fixed positions for at least some nanoseconds. Whether these ionoids could be a sort of precursor to precipitates or even crystals is part of the ongoing investigations of the team.
- Highly Defined, Colloid-Like Ionic Clusters in Solution,

Dennis Kurzbach, Daniel R. Kattnig, Nane Pfaffenberger, Wolfgang Schärtl, Dariush Hinderberger,
ChemistryOpen 2012.
DOI: 10.1002/open.201200025
ChemistryOpen – the first society-owned, open-access, chemistry journal – is a journal of ChemPubSoc Europe published by Wiley-VCH.