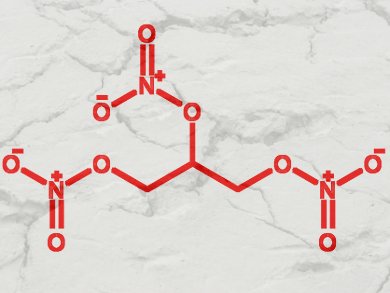
Dynamite is an explosive material consisting of an absorbent substance, such as diatomaceous earth, sawdust, or clay, soaked in nitroglycerin, a shock-sensitive explosive (pictured). It was invented by Alfred Nobel after an accident at a nitroglycerin factory killed his brother.
Nobel discovered that addition of one part diatomaceous earth to three parts nitroglycerin improved the stability of nitroglycerin, allowing it to be handled and transported safely. He also added a small amount of sodium carbonate to neutralize trace quantities of acids that formed during storage. The mixture turned the pure nitroglycerin liquid into a paste which could be shaped into rods of a suitable size for insertion into drilling holes for mining, thus revolutionizing mining.
Nobel patented his discovery in 1867 and later introduced the blasting cap as a detonator which could be ignited by lighting a fuse. The introduction of dynamite greatly improved the safety and reduced the cost of rock blasting, building canals, and demolition.
A stick of dynamite (0.19 kg, 75 % nitroglycerin) contains approximately 1 MJ of energy. Dynamite gains its power from the rapid expansion of the hot gases formed on decomposition of nitroglycerin. Decomposition is triggered by an initial decompression of nitroglycerin, via the blasting cap. Four moles of nitroglycerin produces 35 moles of gases, according to the reaction:
4C3H5N3O9(s)→ 6N2(g) + 12CO(g) + 10H2O(g) + 7O2(g)
It is the speed of decomposition that makes nitroglycerin one of the most powerful explosives. The shock from the initial decomposition reaction creates a supersonic pressure wave that propagates through the nitroglycerin, causing near-instantaneous pressure-induced decomposition of the surrounding fuel in an explosive blast.
Alfred Nobel is the answer to Guess the Chemist (11), which detailed his life.
Also of interest:
- E.-C. Koch on Energetic Metal-Fluorocarbon Materials and Munitions Safety,
Julia Stuthe, Vera Köster,
ChemViews Magazine 2012, June.
DOI: 10.1002/chemv.201200056
Dr. E.-C. Koch, specialist in energetic materials for NATO, talks about the fascination of fireworks and society’s need for explosives - Nobel Prize Trends,
ChemViews Magazine 2012, October.
DOI: 10.1002/chemv.201200110
Which country produced the most Nobel Laureates? How old is a typical Laureate? And is it taking longer for discoveries to be recognized?




I have a saw mill should I be keeping the millings