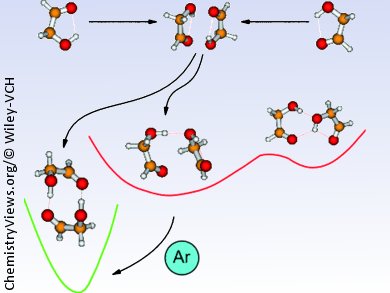
Martin A. Suhm and co-workers, University of Göttingen, Germany, studied the simplest α-hydroxy carbonyl compound, glycolaldehyde, to get an understanding of how carbohydrates can be utilized by nature as most diverse molecular recognition tools.
The authors estimate the energies involved when two internally hydrogen-bonded monomers approach each other. Using supersonic jet infrared and Raman spectroscopy they are able to show that glycolaldehyde preferably forms compact C2-symmetric dimers with intermolecular hydrogen bonds. Further calculations show, that if the geometrical constraints are given, isolated bonds can be favorable over cooperative hydrogen-bond patterns. This could be the origin of the diversity needed for molecular recognition.
- Molecular Recognition in Glycolaldehyde, the Simplest Sugar: Two Isolated Hydrogen Bonds Win Over One Cooperative Pair,
Jonas Altnöder, Juhyon J. Lee, Katharina E. Otto, Martin A. Suhm,
ChemistryOpen 2013, 1(6), 269–275.
DOI: 10.1002/open.201200031
ChemistryOpen – the first society-owned, open-access, chemistry journal – is a journal of ChemPubSoc Europe published by Wiley-VCH.