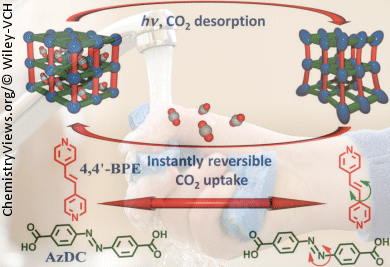
Capture and Release of Carbon Dioxide
In order to reduce the carbon dioxide output from coal power plants, CO2 could be removed from their exhaust (post-combustion capture) and stored or, if possible, used as a carbon source for chemical syntheses. Previous approaches to this have suffered from the fact that they require too much energy. In the journal Angewandte Chemie, Australian scientists have now introduced a new metal–organic framework compound that absorbs CO2 and then releases it upon exposure to sunlight.
Current techniques for the removal of CO2 from coal power plant exhausts by using liquid amines consume vast amounts of energy—sometimes up to 30 % of the energy produced by the plant. Most of the energy consumed in these processes is used to release the CO2 from the absorbent by raising the temperature or applying a vacuum.
A team headed by Richelle Lyndon and Matthew R. Hill is focusing on the use of concentrated sunlight as an alternative energy source for the release of CO2. The Australian researchers hope to achieve this by using metal–organic frameworks (MOFs) to absorb the CO2. MOFs are crystals constructed like a scaffold with pores that can hold guest molecules. The “joints” of the framework consist of metal ions or clusters; the “struts” are organic molecules. Clever selection of the individual components allows the size and chemical properties of the pores to be tailored for specific applications. In this case, they are arranged so that CO2 can be stored in the pores.
Oscillating Structural Changes with UV Light
The team from the Commonwealth Scientific and Industrial Research Organization (CSIRO) and Monash University, Australia, chose to use two different organic molecules for the vertical and horizontal struts. However, the molecules have one thing in common: irradiation with UV light causes them to alter their spatial structure. The molecules are securely fastened into the framework, which results in strain that limits the molecules to moving rapidly back and forth. Because of this, only small, limited regions of the framework move at any one time, and stop the entire structure collapsing.
The oscillating structural changes reduce the attractive forces between the surface of the pores and the absorbed CO2. A majority of the CO2 is squeezed out of the framework like water from a wrung-out sponge.
This process works best with UV light, but also works with concentrated natural sunlight. These light-reactive metal–organic frameworks could thus be an interesting approach for the energy-efficient removal of CO2 from combustion gases. Further investigations are needed to demonstrate how this separation works with real exhaust gases.
- Dynamic Photo-Switching in Metal–Organic Frameworks as a Route to Low-Energy Carbon Dioxide Capture and Release,
Richelle Lyndon, Kristina Konstas, Bradley P. Ladewig, Peter D. Southon, Cameron J. Kepert, Matthew R. Hill,
Angew. Chem. Int. Ed. 2013.
DOI: 10.1002/anie.201206359