This year marks the 50th anniversary of the first patent describing the use of supercritical fluid extraction by Kurt Zosel, Max Planck Institute for Coal Research, Mülheim an der Ruhr, Germany. It also marks the 40th anniversary of Zosel’s US patent of the use of supercritical fluid extraction in the decaffeination of coffee.
Supercritical fluid extraction utilizes a gas that has been pressurized and heated beyond its critical points so that it becomes a supercritical fluid (SCF), that is, a phase of matter where distinct liquid and gas phases do not exist (see Fig. 1).
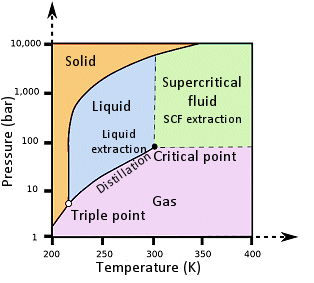
Figure 1. Phase diagram for CO2.
SCFs have low viscosities and no surface tension, which means they are capable of effusing through solids, similar to a gas. Once the SCF has penetrated the pores of the solid, it can selectively dissolve substances contained within the solid, similar to a liquid. The dissolved substances are then carried out of the solid in the SCF and can be recovered through depressurizing the SCF so that it returns to the gas phase, depositing the extracted substance. This results in little or no solvent residues contaminating the extracted product. The gas can then be returned to the SCF phase and reused.
An additional advantage of SCF extraction is the speed at which the extraction can be performed due to the higher diffusivities and lower viscosities of SCFs compared with liquids.
The technique has been applied in the food industry to remove fats and oils from vegetable and animal matter, such as cocoa butter from cocoa beans, soybean oil from soybeans, and essential oils from spices. The technique is most widely used, however, in the decaffeination of coffee through the extraction of caffeine from green coffee beans with supercritical CO2. This process can selectively reduce the amount of caffeine from up to 3 % to under 0.02 % without removing any of the substances that contribute to the aroma formed on roasting.

Kurt Zosel is the answer to Guess the Chemist 15, which gave details about Zosel’s life.
Image © Marc Genovese, www.mgenovese.it.
Source
- Separation with Supercritical Gases: Practical Applications,
K. Zosel,
Angew. Chem. Int. Ed. 1978, 17(10), 702–709.
DOI: 10.1002/anie.197807021
Also of Interest
- Espresso – A Three-Step Preparation,
Klaus Roth,
ChemViews Magazine 2010, May.
DOI: 10.1002/chemv.201000003
Klaus Roth proves that no culinary masterpiece can be achieved without a basic knowledge of chemistry
This and other scientific milestones can be found in:
- Meilensteine der Chemie 2013,

Horst Remane and Wolfgang Girnus,
Nachrichten aus der Chemie 2013, 61(1), 11–22.
DOI: 10.1002/nadc.201390001




