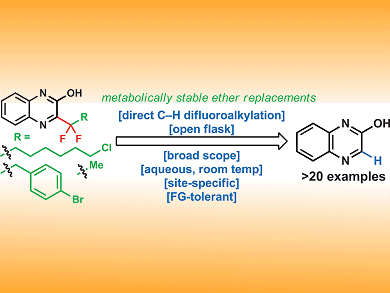
Phil S. Baran and a team at The Scripps Research Institute, La Jolla, USA, and Tel-Aviv University, Israel, have developed a rapid and direct method for the synthesis of fluorinated bioisosteres. Aryl ethers are used in medicinal chemistry, however, they are rapidly metabolized and their replacement with more metabolically stable bioisosteres, such as fluorinated mimics of alkoxy ethers, is an important research target. Previously explored routes involved the conversion of ketones to difluoroalkyl groups but were synthetically cumbersome.
Baran´s team investigated the use of sulfinate salts for a direct approach to this problem, and showed that the new compound sodium difluoroethylsulfinate (DFES-Na), which was prepared by methylation of Hu’s reagent (difluoromethyl 2-pyridyl sulfone) and subsequent cleavage, can be used in direct alkylation reactions. Screening of the reaction conditions showed that ZnCl2 and p-toluenesulfonic acid were the optimal additives. This reaction was then investigated on a wide range of heterocycles, and was shown to be both regioselective and functional-group-tolerant, and can be carried out open to air and in the presence of water. The team also showed that the sodium salt arising from the alkylation of Hu’s reagent with more complicated groups and subsequent cleavage could also be used to functionalize heterocycles.
This method offers a simple and direct method for the synthesis of fluorinated bioisoteres and has the potential to become a standard tool in medicinal chemistry.
- Direct Synthesis of Fluorinated Heteroarylether Bioisosteres,
Qianghui Zhou, Alessandro Ruffoni, Ryan Gianatassio, Yuta Fujiwara, Eran Sella, Doron Shabat, Phil S. Baran,
Angew. Chem. Int. Ed. 2013.
DOI: 10.1002/anie.201300763