Eutrophication, which is caused by the accumulation of excess nutrients in natural water bodies, has become a serious environmental problem. The overgrowth of algae and the depletion of dissolved oxygen diminish water quality and reduce biodiversity. Decreasing the concentration of phosphorus species in water bodies is essential to control eutrophication and recover phosphorous, which is a depleting natural resource, and various methods have been developed for their removal. The adsorption of phosphates by using functional adsorbents is a promising approach owing to its simple operation and reduced sludge production compared with the established method of chemical precipitation.
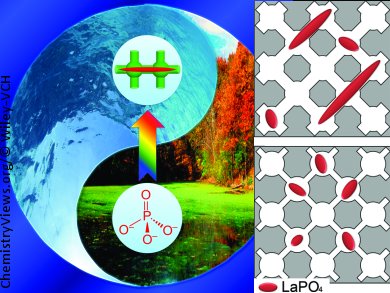
Chengzhong Yu and co-workers, University of Queensland, Australia, have systematically investigated the behavior of chemisorbed phosphates inside 3D cubic mesoporous materials with adjustable structural parameters by using lanthanum-functionalized cage-type mesoporous materials with tuneable entrance sizes. With small entrances (>5 nm), chemisorbed phosphates are confined in isolated cages, resulting in low La usage efficiency and poor adsorption performance. For materials with large entrances, phosphate nanorods can be present in both cages and entrances (pictured), increasing lanthanum usage efficiency and enhancing phosphate-adsorption performance. However, as the La loading ratio increases, LaPO4 nanorods that penetrate connected cages can block the entrances, preventing the diffusion of phosphate ions through the entrances and resulting in low adsorption capacity and slow adsorption rates.
The study provides new insights into the rational design of phosphate adsorbents with controlled structures and high performance.
- Confinement of Chemisorbed Phosphates in a Controlled Nanospace with Three-Dimensional Mesostructures,
Jie Yang, Liang Zhou, Jun Zhang, Jin Zou, Zhiguo Yuan, Chengzhong Yu,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201300273