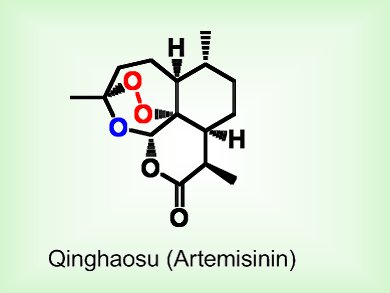
Qinghaosu, or artemisinin, is the most effective antimalarial drug available today because it is fast acting and the malaria parasite has not yet developed resistance to it. However, the total synthesis of qinghaosu is particularly difficult. The most prominent feature of the compound, and the most difficult to incorporate, is the 1,2,4-trioxane and peroxy bridge that is responsible for its antimalarial activity.
Yikang Wu and colleagues at the Shanghai Institution of Organic Chemistry, China, the Swiss Tropical and Public Health Institute, Basel, and the University of Basel, Switzerland, have developed a new synthetic route to qinghaosu and analogues that show good antimalarial activity.
With the aid of a molybdenum catalyst, the stereoselective perhydrolysis of a sterically hindered epoxide ring was achieved at ambient temperature in ethereal H2O2. The resulting hydroperoxide was readily converted into a 1,2,4-trioxane, from which qinghaosu and analogues were constructed. By varying the relative configuration and functionality of the peroxy substrate, some insight into the stereochemical requirements of the key perhydrolysis reaction was also gained.
Some of the new trioxane compounds showed ng mL–1 level in vitro activity against the Plasmodium falciparum parasite that is comparable to, or better than, that of the existing antimalarial drugs chloroquine and artesunate. These potent new analogues and their activity data provide valuable information about structure–activity relationships for the trioxane-type antimalarials and may serve as useful lead compounds.
- Potent Antimalarial 1,2,4-Trioxanes through Perhydrolysis of Epoxides,
Hong-Dong Hao, Sergio Wittlin, Yikang Wu,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201300076