High Yield: Cell-free Enzyme Cascade Makes Hydrogen from Xylose
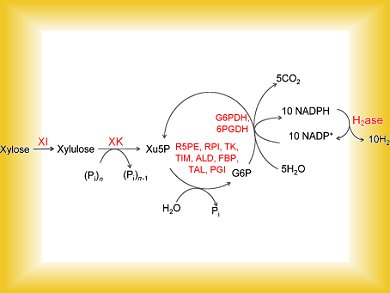
Fuel cells are a highly promising means of producing electricity. However, the hydrogen they require is still largely obtained from coal, oil, or natural gas. Producing hydrogen from less expensive biomass is an attractive alternative, but has not produced sufficient yields to date. In the journal Angewandte Chemie, a team of American and Mexican researchers has now introduced a cell-free biosystem of thirteen enzymes that can produce hydrogen from xylose, one of the main components of plants, in yields of over 95 %.
Xylose is a pentose (a sugar molecule containing five carbon atoms), and is one of the main building blocks of lignocellulosic biomass—wood and parts of woody plants. It is not economically feasible to separate xylose from the other components of biomass for the production of hydrogen. There are microorganisms that can convert xylose and glucose, the building block that makes up cellulose, into hydrogen. However, the yields are very low.
Y.-H. Percival Zhang, Virginia Tech, Blacksburg, USA, and his co-workers in the USA and Mexico have thus resorted to a trick: They are using the enzymes used by the microorganisms, but in a cell-free system. They combined thirteen enzymes and various cofactors like NADPH into a complex cascade that do not exist in the natural metabolic systems. In a bioreactor, they were able to produce hydrogen from xylose with a yield of over 95 %.
Xylulokinase from Thermotoga Maritima for Xylose Activation
The downside: In the first step of the reaction, xylose is isomerized into xylulose, which must be activated in a second step by addition of a phosphate group. This requires ATP (adenosine triphosphate), the “energy carrier” of cells, to “pump” chemical energy into the enzyme cascade. Unfortunately, ATP is a very expensive material. The thing that depends on ATP is the splitting of the energy-rich bonds between individual phosphate groups. The researchers thus had an idea: They wanted to replace the ATP with a more economical substance, polyphosphate, which also contains energetic phosphate bonds. However, this requires a xylulokinase, an enzyme that attaches phosphate groups to xylulose, and can use polyphosphate instead of ATP.
Polyphosphate is found in volcanic rocks and in deep-oceanic steam vents. Primeval organisms may have used this substance. The researchers isolated the gene for a xylulokinase from thermotoga maritima, a thermophilic microorganism found in such environments, and used genetic engineering to produce the enzyme. As they hoped, this enzyme can also use polyphosphate and can successfully replace the ATP-dependent xylulokinase in the enzyme cascade.
This team had previously developed a synthetic enzymatic route for the production of hydrogen from cellulose. Now both of the major components of biomass, cellulose and xylose, can be converted together in a new approach for the more economical production of hydrogen.
- High-Yield Production of Dihydrogen from Xylose by Using a Synthetic Enzyme Cascade in a Cell-Free System
Julia S. Martín del Campo, Joseph Rollin, Suwan Myung, You Chun, Sanjeev Chandrayan, Rodrigo Patiño, Michael W. W. Adams, Y.-H. Percival Zhang,
Angew. Chem. Int. Ed. 2013.
DOI: 10.1002/anie.201300766