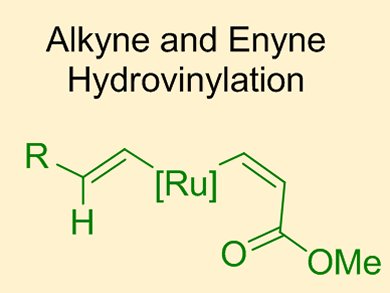
Previous work from Bernd Plietker and co-workers, University of Stuttgart, Germany, has focused on the ruthenium-catalyzed hydrovinylation of internal alkynes for the formation of 1,3-dienes (green catalytic cycle below). One limitation of this original process was a competing catalytic pathway which resulted in the unwanted homocoupling of two alkyne substrates to give the corresponding enyne (red catalytic cycle).
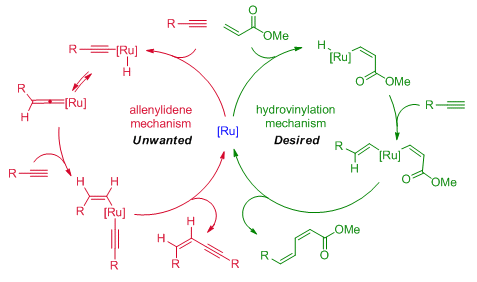
In their latest report, the group show how careful control of the reaction conditions can suppress the formation of the homocoupled product. The key modifications were the use of an excess of the acrylate coupling partner and microwave irradiation. Using these modified conditions, 1,3-dienes were obtained in good yields with good E/Z selectivities. In addition, the reaction could also be extended to terminal enynes such as 2 for the formation of 1,3,5-trienes.
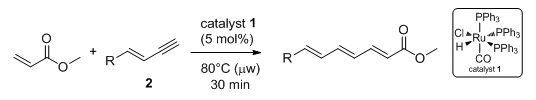
The formation of these highly functionalized molecules is likely to be useful in further synthetic studies.
- Microwave-Accelerated Ru-Catalyzed Hydrovinylation of Alkynes and Enynes: A Straightforward Approach toward 1,3-Dienes and 1,3,5-Trienes,
Tobias Schabel, Bernd Plietker,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201300790