Tridentate or “pincer” ligands are a versatile class of donors for transition metals. They are extremely stable, because of their multidentate nature, and they can accommodate a great variety of combinations of donor atoms, leading to a wide range of products. One interesting use of such ligands is small molecule activation. Electron-rich, strongly donating pincer ligands can labilize ligands trans to the central atom donor and stabilize high-oxidation-state intermediates.
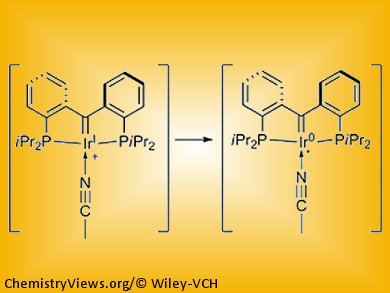
Warren Piers and co-workers, University of Calgary, Alberta, Canada, report the reductive coupling of acetonitrile mediated by an iridium(I) complex with an electron-rich tridentate pincer carbene ligand (pictured above). The mechanism of this reaction (pictured below) is unusual for a late transition metal. It results from the extraordinarily electron-donating nature of the pincer carbene ligand.
The reductive coupling of a coordinated acetonitrile ligand is mediated by one electron per iridium center and leads to a dimer bridged by a diiminato ligand derived from two molecules of CH3CN (see below, right).
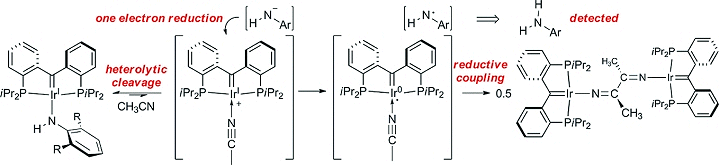
The unusual reactivity observed opens up possibilities of application in catalysis.
- Acetonitrile Coupling at an Electron-Rich Iridium Center Supported by a PCsp²P Pincer Ligand,
Richard J. Burford, Warren E. Piers, Masood Parvez,
Eur. J. Inorg. Chem. 2013.
DOI: 10.1002/ejic.201300152