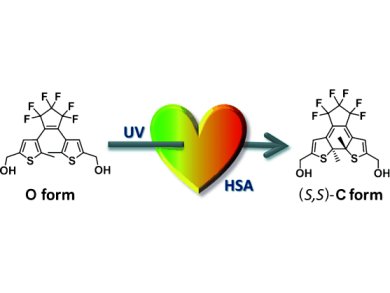
The application of photochromic compounds to biological systems has recently attracted attention. Photochromic diarylethenes (DAEs) switch, based on their 6π-electrocyclization-cycloreversion reactions, between a 1,3,5-hexatriene and a 1,3-cyclohexadiene. The less-water-soluble photochromic compounds can be delivered to cells in living creatures by using serum albumin as the carrier.
Yasushi Yokoyama and co-workers, Yokohama National University, Japan, have successfully performed enantioselective photochromic ring-closing reactions of three bisthienylethene compounds containing either no or two hydroxyl groups in aqueous media by taking advantage of the hydrophobic pockets in human serum albumin (HSA). Thus, as shown in the picture, a bisthienylperfluorocyclopentene with a hydroxyl group at each end of the molecule, incorporated in the HSA pocket, yields the colored (S,S) cyclic form in 63 % ee at 25 °C and 71 % ee at –4 °C upon irradiation with 313 nm light.
- Enantioselective Photochromism of Diarylethenes in Human Serum Albumin,
M. Fukagawa, I. Kawamura, T. Ubukata, Y. Yokoyama,
Chem. Europ. J. 2013.
DOI: 10.1002/chem.201301459