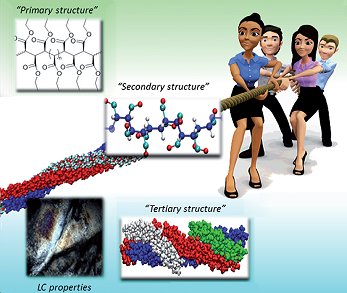
The supramolecular assembly of synthetic macromolecules into complex helical aggregates drastically alters their properties, potentially providing new classes of material with unique functionality caused by the “tertiary structure”. Synthetic helical assemblies of macromolecules could play a role in the development of liquid-crystalline polymers (LCPs), which have important potential applications, for example, in organic photovoltaic materials and as actuators. LCPs generally contain segments of sp2-hybridized carbon atoms to provide sufficient rigidity to the macromolecular chains to form a liquid-crystalline phase. This rigidity can also be induced by formation of helical assemblies that lock the polymer chains in a specific, rigid conformation, thus allowing the formation of LCPs from sp3-carbon-based polymers; however, few examples have been reported.
Bas de Bruin and co-workers, Universiteit van Amsterdam, the Netherlands, have described the self-assembly of syndiotactic polycarbene polymers of poly(ethylidene acetate) (st-PEA) into van der Waals stabilized LC aggregates. The formation of these LC aggregates from such flexible sp3-carbon-backbone synthetic polymers is unexpected. A combination of experimental and molecular-mechanics-based molecular dynamics studies reveals that this surprising behavior is due to self-aggregation of st-PEA into triple-helix aggregates.
This finding demonstrates the importance of the “tertiary structure” of synthetic polymers to their material properties.
- On the “Tertiary Structure” of Poly-Carbenes; Self-Assembly of sp3-Carbon-Based Polymers into Liquid-Crystalline Aggregates,
Nicole M. G. Franssen, Bernd Ensing, Maruti Hegde, Theo Dingemans, Ben Norder, Stephen J. Picken, Gert O. R. Alberda van Ekenstein, Ernst van Eck, Johannes A. A. W. Elemans, Mark Vis, Joost N. H. Reek, Bas de Bruin,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201301403