The carbon compounds called “carbones”, L→C←L, have only recently been discovered. These compounds possess two lone-pair orbitals at a dicoordinated carbon atom, thus making them double donors. This new bonding mode has inspired the synthesis of several new compounds. For example, carbodiphosphorane (PPh3)2C has been shown to stabilize the previously elusive borenium cation, BH2+, in the complex [(PPh3)2C→BH2]+.
Gernot Frenking and co-workers, Philipps-Universität, Marburg, Germany, explored carbones in a joint theoretical and experimental study to gain a better understanding of these compounds. The theoretical calculations predict that carbodiphosphorane (PPh3)2C can stabilize cations other than BH2+ by the formation of complexes such as dication [(PPh3)2C→CH2]2+ and even trication [(PPh3)2C→NH2]3+. This approach can not be extended to the tetracation [(PPh3)2C→OH2]4+ as this species dissociates to the trication [(PPh3)2C→OH]3+.
.jpg)
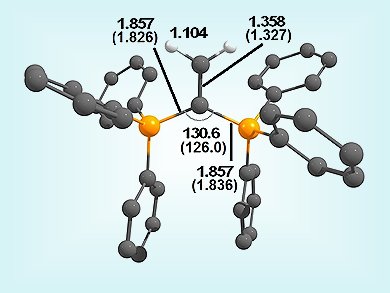
The prediction that [(PPh3)2C→CH2]2+(pictured) could be formed was verified by the synthesis and isolation of this dication. However, analysis of the bonding suggests that the compound is better described as the substituted olefin [(PPh3)2C=CH2]2+.
- Exploiting the Twofold Donor Ability of Carbodiphosphoranes: Theoretical Studies of [(PPh3)2C→EH2]q (Eq=Be, B+, C2+, N3+, O4+) and Synthesis of the Dication [(Ph3P)2C=CH2]2+,
Mehmet Ali Celik, Gernot Frenking, Bernhard Neumüller, Wolfgang Petz,
ChemPlusChem 2013.
DOI: 10.1002/cplu.201300169