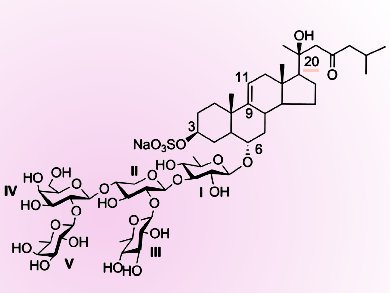
Steroidal glycosides are considered to be responsible for the general toxicity of starfish. They also show a broad spectrum of pharmacological activities.
Usually they are divided into three substructural types: asterosaponin (sulfated steroidal glycosides), steroidal cyclic glycosides, and polyhydroxysteroidal glycosides. Thornasteroside A (pictured) is the first asterosaponin isolated from Acanthaster planci L. in 1978. Its total synthesis is a challenge. One of the obstacles is the presence of the 20-OH group β to a carbonyl group in the aglycon.
Yingxia Li and colleagues, Fudan University, Shanghai, China, have synthesized the pentasaccharide moiety of thornasteroside A by using a [3+1+1] strategy.
A galactopyranosyl donor equipped with a neighboring participating Lev (levulinoyl) group at the 2-position was first coupled with a trisaccharide acceptor to construct the β(1→4) glycosidic bond. Then the Lev group was selectively removed, and subsequent glycosylation with a perbenzoylated D-fucopyranosyl Schmidt donor efficiently gave the desired pentasaccharide.
The work significantly facilitates the total synthesis of the whole thornasteroside A molecule and also of other structurally related oligosaccharides isolated from starfish and other marine organisms.
- Synthesis of the Pentasaccharide Moiety of Thornasterside A,
Junlong Xiong, Zhichao Lu, Ning Ding, Sumei Ren, Yingxia Li,
Eur. J. Org. Chem. 2013.
DOI: 10.1002/ejoc.201300575