Transition-metal-catalyzed cyclization of 1,6-enynes is a powerful method for constructing carbo- and heterocyclic molecules. Cyclization of 1,6-enynes with main-group organometallics or metal hydrides is particularly attractive because these reactions offer new approaches for the highly stereoselective formation of ring systems with exocyclic tri- or tetrasubstituted C–C double bonds. Despite developments in this area, the use of inexpensive metal catalysts for these reactions remains in high demand.
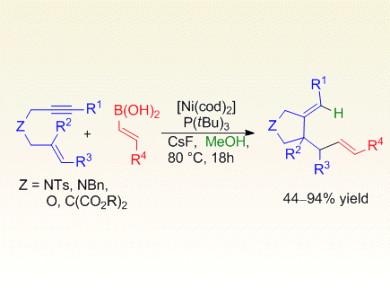
Chien-Hong Cheng and co-workers, National Tsing Hua University, Taiwan, describe a low-cost, highly chemo- and stereoselective nickel-catalyzed tandem cyclization/coupling of electronically unactivated 1,6-enynes with alkenyl boronic acids to provide various substituted pyrrolidines and dihydrofurans, which are found in a variety of natural products and biologically active molecules.
A variety of alkenyl boronic acids were successfully employed and elucidation of the reaction mechanism provided evidence for chemoselective protonation of the nickelacyclopentene intermediate leading to the addition of the alkenyl group of the boronic acid at the alkene terminus of the 1,6-enyne in the final product. Further studies to expand the scope of the tandem cyclization are currently underway.
- Nickel-Catalyzed Chemo- and Stereoselective Alkenylative Cyclization of 1,6-Enynes with Alkenyl Boronic Acids,
C.-M. Yang, S. Mannathan, C.-H. Cheng,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201302180