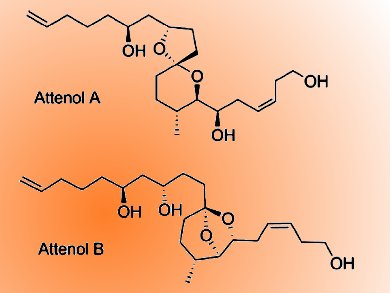
Attenols A and B are a novel class of spiroacetal ether compounds isolated from the Chinese bivalve Pinna attenuata (pictured). They have attracted the attention of several synthetic organic chemists, not only for their structural complexity but also because of their biological significance.
Jhillu S. Yadav and colleagues, Indian Institute of Chemical Technology (CSIR), Hyderabad, India, have developed a highly convergent route to synthesize attenols A and B in a highly stereoselective manner. The strategy in the total synthesis of these compounds involves mainly the construction of the spiroacetal core from tosylmethyl isocyanide (TosMIC). The diastereoselective creation of the methyl center was achieved by reductive cyclization and a consecutive Prins cyclization followed by a reductive opening cascade for anti-1,3-diol motifs. The total synthesis proceeded in 15 steps with 15.4% overall yield.
- Stereoselective Total Synthesis of Attenols A and B,
Jhillu S. Yadav, Poli Adi Narayana Reddy, Yerabolu Jayasudhan Reddy, Syeda Meraj, Attaluri R. Prasad,
Eur. J. Org. Chem. 2013.
DOI: 10.1002/ejoc.201300623