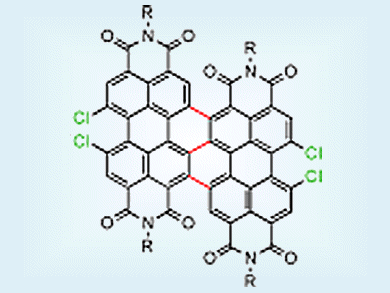
Conjugated triply linked perylene-3,4:9,10-tetracarboxylic acid bisimide dimers (diPBIs; pictured) behave as excellent n-type transistors and are highly air-stable because of their low-lying lowest unoccupied molecular orbital (LUMO), as well as their dense packing structure. However, the synthesis of di(PBIs) is normally inefficient and requires a large excess of ligands to achieve dimerization.
Wenping Hu and co-workers, Institute of Chemistry, Chinese Academy of Sciences, have developed a ligand-free copper-promoted dimerization of PBIs. Their method involves treating the PBIs with a copper(II) salt and potassium tert-butoxide at 90°C. This combination of C–C homocoupling and C–H activation affords fully conjugated, triply linked di(PBIs) in double the yield than was previously achievable.
The researchers hope that this new technique will enrich the synthetic chemistry of PBIs and other condensed ring systems, and might help in the development of air-stable n-type semiconducting materials for cost-effective organic circuitry.
- A Ligand-free Copper-promoted Dimerization of Perylene Bisimide by Aromatic C–C Homocoupling and C–H Activation,
Xiuqiang Lu, Huanli Dong, Ping He, Xiaotao Zhang, Jie Liu, Qing Meng, Lang Jiang, Zhaohui Wang, Yonggang Zhen, Wenping Hu,
Asian J. Org. Chem. 2013, 2, 558–560.
DOI: 10.1002/ajoc.201300109