The donor–acceptor bond in Lewis acid–base adducts of group 13 organometallic compounds and the chalcogen atoms S and Se is relatively weak, as these chalcogen atoms are only moderate donors and the organic groups at the metal atom diminish their acceptor ability.
Harald Krautscheid and colleagues, Universität Leipzig, Germany, synthesized molecular compounds with a copper–chalcogen–indium binding motif and characterized them crystallographically. The reaction of [(iPr3P)nCuESiMe3] with the Lewis acidic InMe3 did not result, as expected, in cleavage of the chalcogen–silicon bond but in the formation of a simple Lewis acid–base adduct. These lewis acid–base adducts of trimethyl-indium and phosphane-stabilized copper(I)(trimethylsilyl)chalcogenolates are very unstable under atmospheric conditions and decompose at ambient temperatures.
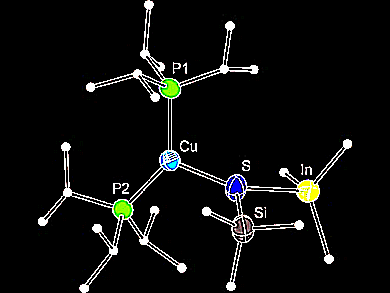
Adducts E = S (pictured) and E = Se have the rare structural feature of a chalcogen donor atom with (almost) planar coordination. This is a consequence of the steric demand of the bulky phosphane ligands that coordinate the copper atom. The team could support this explanation by DFT calculations.
- Synthesis and Crystal Structures of [(iPr3P)2Cu(μ-ESi-Me3)(InMe3)] (E = S, Se): Lewis Acid–Base Adducts with Chalcogen Atoms in Planar Coordination,
Ralf Biedermann, Oliver Kluge, Daniel Fuhrmann, Harald Krautscheid,
Eur. J. Inorg. Chem. 2013.
DOI: 10.1002/ejic.201300768