The functionalization of the framework of pentacenes and pentacenequinones has, to date, focused on organic substituents. It is accomplished mainly by appending alkyl, aryl, and alkyne residues to the framework to influence the HOMO–LUMO gap and packing motif in the crystalline state. The organometallic chemistry of pentacenes has been little explored, and organometallic derivatives of pentacenequinone have been unknown.
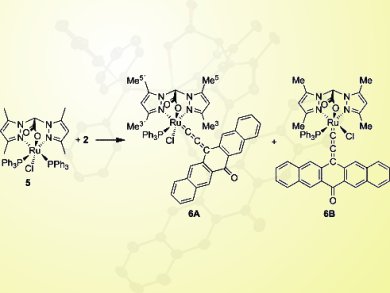
Nicolai Burzlaff, University of Erlangen-Nürnberg, Erlangen, Germany, and colleagues report the first synthesis of ruthenium–allenylidene complexes with a pentacenequinone-based ligand. [Ru(bdmpza)Cl(PPh3)2] (5) was prepared according to the literature, and addition of 13-ethynyl-13-hydroxypentacen-6-one (2) led to the formation of two structural isomers, namely 6A and 6B (pictured).
The complexes show an electronic absorption in the NIR region that arises from forbidden metal-to-ligand charge-transfer (MLCT) transitions, as the team explained by DFT calculations.
The arrangement observed for 6B in the crystalline state renders the presented complexes promising candidates for metal-tuned field-effect transistors (FETs) or “organic” metal–semiconductor field-effect transistors (OMESFETs). Their electron-accepting ability and low-energy absorption characteristics might be tuned for use in solar cells. Both aspects present an appealing starting point for new kinds of functionalized organic semiconductors.
- Allenylidene Complexes Based on Pentacenequinone,
Frank Strinitz, Andreas Waterloo, Johannes Tucher, Eike Hübner, Rik R. Tykwinski, Nicolai Burzlaff,
Eur. J. Inorg. Chem. 2013.
DOI: 10.1002/ejic.201300759