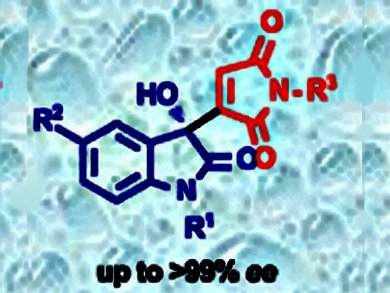
Maleimide derivatives are excellent electrophiles, dienophiles and dipolarophiles for the asymmetric synthesis of chiral succinimide derivatives. However, application of maleimides as a nucleophile partner in the asymmetric transformations was not known.
Pankaj Chauhan and Swapandeep Singh Chimni, Guru Nanak Dev University, Amritsar, India, have reported an organocatalytic enantioselective Morita–Baylis–Hillman (MBH) reaction that involves maleimides as nucleophiles. The enantioselective procedure for the organocatalytic MBH reaction of maleimides with isatins is catalyzed by the readily available chiral β-isocupreidine (β-ICPD). A series of optically active 3-substituted-3-hydroxyoxindole derivatives were synthesized in up to 96 % yield and up to >99 % ee from a diverse range of maleimides and isatin derivatives under mild reaction conditions.
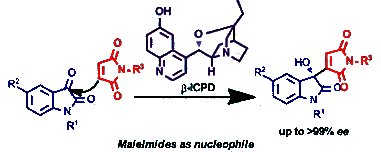
This new method of using maleimides as nucleophiles in asymmetric reactions could initiate the further use of maleimides as nucleophiles in related stereoselective transformations to provide valuable chiral molecules.
- Organocatalytic Enantioselective Morita-Baylis-Hillman Reaction of Maleimides with Isatins,
Pankaj Chauhan, Swapandeep Singh Chimni,
Asian J. Org. Chem. 2013, 2, 586–592.
DOI: 10.1002/ajoc.201300093