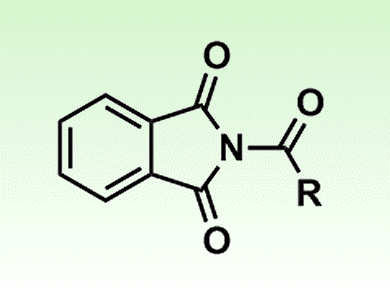
Amide compounds have rarely been used as synthetic intermediates because the amide C–N bond is unreactive. However, it has been shown that a transition-metal-catalyzed method allows the use of N-acylphthalimides, moderately reactive amide derivatives, for the synthesis of acid fluorides and thioesters. Acid halides can be obtained from the amides under mild conditions. This contraintuitive chemical transformation was attained by shifting the metal-catalyzed equilibrium to the product in the presence of a nitrogen-atom-trapping reagent.
The team behind the development, led by Masahiko Yamaguchi of Tohoku University, Japan, showed that the rhodium-catalyzed method can be used for the synthesis and reaction of N-acylphthalimides. N-Acylphthalimides, thioesters, and acid fluorides are equilibriated under rhodium catalysis, and the equilibrium was shifted in either direction in the presence of appropriate heteroatom-trapping reagents.
The team intends to develop this technique into novel organoheteroatom manipulation methods for organic synthesis.
- Rhodium-Catalyzed Synthesis and Reactions of N-Acylphthalimides,
Guangzhe Li, Mieko Arisawa, Masahiko Yamaguchi,
Asian J. Org. Chem. 2013.
DOI: 10.1002/ajoc.201300094