Separating Polymorphs
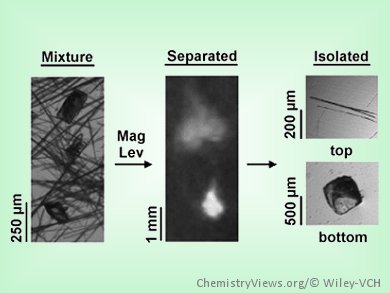
The effectiveness of crystalline pharmaceuticals is not only influenced by molecular composition; the structure of the crystals is also important because it determines both the solubility and the rate of dissolution, which in turn affect the bioavailability. Researchers from Cambridge, Massachusetts, USA, have recently developed a method by which different crystals can be separated by their density in a magnetic field. In the journal Angewandte Chemie, they have now demonstrated the extraordinary efficiency of separation through “magnetic levitation”.
Many organic substances crystallize in multiple crystal structures known as polymorphs. Drugs are not the only class of products for which this can lead to problems. Different crystal structures can lead to color variation in pigments and dyes; in explosives it can lead to changes in sensitivity
It is not always possible to control the crystallization process to obtain only the desired polymorph. Clean separation is often difficult, and occurs either by chance or through long and complex procedures. A team led by Allan S. Myerson at the Massachusetts Institute of Technology (MIT) and George M. Whitesides at Harvard University has recently developed a simple method that makes it possible to separate polymorphs conveniently and reliably within minutes through magnetic levitation. The technique is based on the fact that different crystal modifications almost always have different densities.
Suspended Crystals
Their clever method works like this: Two magnets are placed one over the other at 4.5 cm apart with like poles facing. This produces a magnetic field with a linear gradient and a minimum in the middle, between the two magnets. The crystals to be separated are suspended in a solution of paramagnetic ions and placed in a tube within the magnetic field. The gravitational force causes the crystals to sink down to the bottom of the tube. By doing so, a crystal “displaces” its own volume of the paramagnetic fluid “upwards”. Yet, this is unfavorable, because the paramagnetic fluid is attracted by the magnet — the attraction gets stronger closer to the face of the magnet. The crystal sinks as long as it reaches a distance above the magnet where the gravitational force and the magnetic attraction on the equivalent volume of the paramagnetic fluid are balanced. At this point, the crystal will “float” in the fluid. As the strength of the gravitational force depends on the density of the crystal, the “floating point” is different for different crystal modification. The solution is then removed from the tube with a cannula and divided into multiple fractions.
Through separation of different polymorphs of 5-methyl-2-[(2-nitrophenyl)amino]-3-thiophencarbonitrile, sulfathiazole, carbamazepine, and trans-cinnamic acid, the scientists have presented impressive evidence of the efficiency of their new technique, which allows for the separation of crystal forms with a difference in density as low as 0.001 g/cm3.
- Using Magnetic Levitation to Separate Mixtures of Crystal Polymorphs,
Manza B. J. Atkinson, David K. Bwambok, Jie Chen, Prashant D. Chopade, Martin M. Thuo, Charles R. Mace, Katherine A. Mirica, Ashok A. Kumar, Allan S. Myerson, George M. Whitesides,
Angew. Chem. Int. Ed. 2013.
DOI: 10.1002/anie.201305549