Pramlintide (Symlin), a synthetic version of amylin, is a 37-amino acid peptide that is co-secreted with insulin by pancreatic β-cells. It was developed and approved in 2005 by the FDA for use in US patients with type I and II diabetes in conjunction with the administration of prandial insulin to improve postprandial glycemic control. Pramlintide, however, still has significant limitations, typical of other peptide drugs. These include low circulatory half-life and poor solubility/bioavailability. This means that it currently has to be administered as a separate injection to insulin. Glycosylation of several synthetic peptides relevant to the treatment of diabetes has been attempted to improve their pharmacokinetic properties, including insulin, GLP-1, and exendin-4. Glycosylation of amylin or amylin analogues, such as pramlintide may, therefore, be expected to produce glycopeptides with superior properties.
Antony J. Fairbanks and co-workers, University of Canterbury, New Zealand, have synthesized glycosylated analogues of pramlintide, by a combination of solid-phase peptide synthesis and highly efficient enzymatic glycosylation.
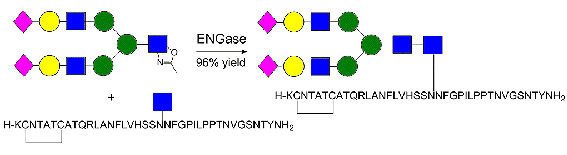
The synthetic peptides displayed both in vitro and in vivo activity as amylin receptor agonists. They were similar in moderating the rise in blood glucose during an oral glucose tolerance test (OGTT) in Sprague Dawley (SD) rats. The effects of glycosylation on other pharmacokinetic properties of these peptides are currently in progress and such studies may produce glycopeptide materials that display advantageous properties relative to pramlintide, and which may, therefore, find clinical application for the treatment of type I and II diabetes in conjunction with insulin.
- Glycosylation of Pramlintide: Synthetic Glycopeptides that Display In Vitro and In Vivo Activities as Amylin Receptor Agonists,
Yusuke Tomabechi, Guy Krippner, Phillip M. Rendle, Marie A. Squire, Antony J. Fairbanks,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201303303




