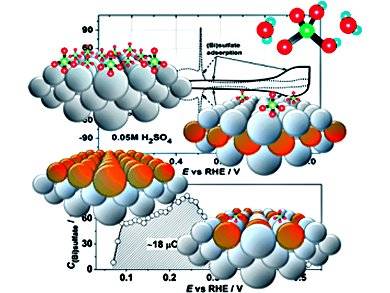
Selective positioning of monolayer amounts of foreign atoms at the surface and subsurface regions of metal electrodes is a promising way to fine-tune the properties of the electrode/electrolyte interface. Jakub Tymoczko, Wolfgang Schuhmann, and Aliaksandr S. Bandarenka, Ruhr-Universität Bochum, Germany, have demonstrated a promising and relatively easy route of tuning the properties of electrified interfaces between Pt(111) electrodes and sulfate-containing electrolytes.
They used model Pt(111) single-crystal electrodes to demonstrate how the relative position of Cu atoms at the surface drastically changes the adsorption energies for (bi)sulfate anions. Cyclic voltammetry, electrochemical impedance spectroscopy (EIS) and electrogravimetric measurements performed with electrochemical quartz crystal microbalance (EQCM) were used.
In the case of Cu–Pt(111) surface alloys, specific adsorption of the anions starts earlier compared to the unmodified Pt(111) surface. Placing Cu atoms into the second atomic layer weakens the binding between the surface and the anions. Cu pseudomorphic overlayers do not reveal any specific adsorption of (bi)sulfates within the region of the overlayer stability.
 Position of Cu Atoms at the Pt(111) Electrode Surfaces Controls Electrosorption of (H)SO4(2)– from H2SO4 Electrolytes,
Position of Cu Atoms at the Pt(111) Electrode Surfaces Controls Electrosorption of (H)SO4(2)– from H2SO4 Electrolytes,
Jakub Tymoczko, Wolfgang Schuhmann, Aliaksandr S. Bandarenka,
ChemElectroChem 2013.
DOI: 10.1002/celc.201300107