In the field of nuclear technology, especially with respect to nuclear fuel reprocessing and safe disposal, the study of the covalent nature of uranium–ligand interactions has become extremely important.
Marinella Mazzanti, Laboratoire de Reconnaissance Ionique et Chimie de Coordination, Grenoble, France, and co-workers are interested in arene–uranium inverted-sandwich complexes, that is—complexes in which two metal ions are bridged by an arene in an μ-η6:η6-symmetrical fashion—owing to the presence of π- and δ-covalent interactions between the arene and uranium d and f orbitals.
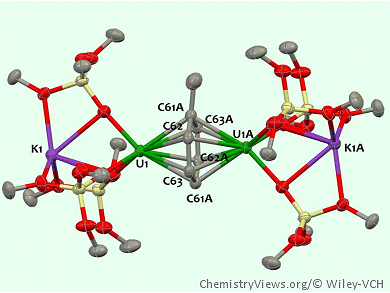
The team have synthesized a family of three toluene-bridged diuranium inverted-sandwich complexes supported by siloxide ligands. The parent arene-bridged complex was selectively reduced, by using stoichiometric amounts of KC8, to its monoanionic and dianionic analogues:
.jpg)
From structural characterization, investigation of the magnetic properties, and computational studies, the team found that all of these complexes are best described as high-valent uranium metal centers bridged by a tetra-anionic (toluene)4– moiety. They also found that in the mono- and dianionic species, the K+ counterions are coordinated to the supporting siloxide ligands.
The reactivity of these systems towards K+ ions leading to a change of the state of charge, is unprecedented in the chemistry of inverted-sandwich complexes and demonstrates the key role of the coordinating counterions for the stability of these complexes.
- Cation-Mediated Conversion of the State of Charge in Uranium Arene Inverted-Sandwich Complexes,
C. Camp, V. Mougel, J. Pécaut, L. Maron, M. Mazzanti,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201302752