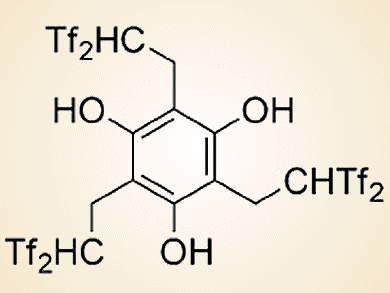
The bis(trifluoromethanesulfonyl)methyl (Tf2CH; Tf = CF3SO2) group is a very strong acidic functionality that has an active hydrogen atom on an electronically neutral carbon atom. Hikaru Yanai and co-workers, Tokyo University of Pharmacy and Life Sciences, Japan, have developed two kinds of acid catalysts on the basis of this carbon acid.
The first one is a triple carbon acid that has three Tf2CH groups in the molecular structure (see picture above). This catalyst was used in the first example of an organocatalytic olefination of arene-fused lactones by reaction with a silicon enolate (see scheme below).
The second one is a zwitterion that contains a highly stabilized carbanion moiety, which is an intermolecular salt between Tf2CH functionality and aniline functionality. By using this zwitterion instead of the triple carbon acid, similar reactions to those conducted with the first catalyst gave aldol adducts in a selective manner (see scheme below). Furthermore, the adducts were successfully converted into highly substituted 1-naphthols, which are common in the structure of biologically active naphthalenes.
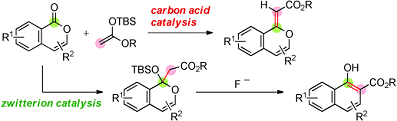
It is hoped that these findings on carbon-acid and zwitterion catalysis will provide the basis for the Tf2CH group to be a useful acidic functionality for molecular design of organocatalysts.
- Organic Acid Catalysts in Reactions of Lactones with Silicon Enolates,
Hikaru Yanai, Nobuyuki Ishii, Takashi Matsumoto, Takeo Taguchi,
Asian J. Org. Chem. 2013.
DOI: 10.1002/ajoc.201300157