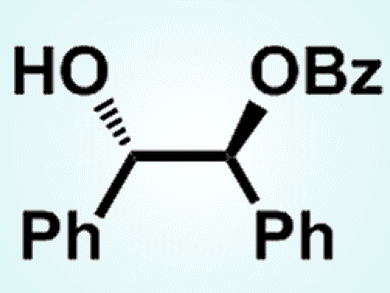
Monohydroxy compounds are usually more reactive than vicinal diol and polyol derivatives. The selective monoacylation of the latter two is also difficult in general. However, LiCl has now been found to specifically activate vicinal diols and polyols instead of isolated monols, and promotes the electrophilic substitution to yield selectively the mono-O-substituted products.
Yutaka Watanabe and co-workers from Ehime University, Japan, showed that diol derivatives capable of forming intramolecular hydrogen bonds are especially reactive in this system:
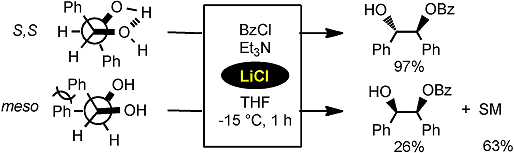
The group hopes that this LiCl-aided substitution of alcohols may be efficiently used to synthesize a variety of polyol and saccharide derivatives of interest.
- Specific Enhancement of Reactivity and Selectivity in the Substitution Reactions of Polyhydroxy Derivatives by Lithium Chloride,
Yutaka Watanabe, Mao Kawamoto, Hiroyuki Shintaku, Kae Tanabe, Hidetoshi Ohta, Minoru Hayashi,
Asian J. Org. Chem. 2013.
DOI: 10.1002/ajoc.201300163