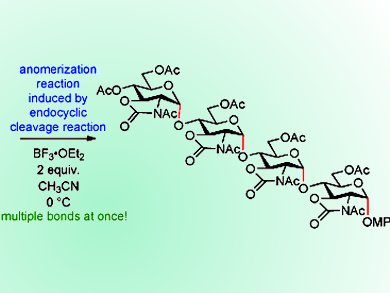
Chemical synthesis of glycosides has contributed greatly to glycobiology, with the glycosylation reaction recognized as one of the most essential reactions in oligosaccharide synthesis. Most 1,2-trans glycosides can be easily prepared by exploiting neighboring-group participation of the 2-acyl protecting group. However, 1,2-cis glycosides are often difficult to synthesize and 1,2-cis aminoglycosides, found in many biologically active oligosaccharides, such as heparin and antibiotics, are particularly difficult to prepare in a completely stereoselective manner.
Shino Manabe, Hiroko Satoh, and co-workers, RIKEN, Saitama, and National Institute of Informatics, Tokyo, both Japan, and colleagues from University of Zurich, Swiss Federal Institute of Technology (ETH), Zurich, and IBM Research Zurich, all Switzerland, have demonstrated through synthetic and computational studies that aminoglycosides containing a 2,3-trans carbamate group easily undergo anomerization from the 1,2-trans glycoside to the 1,2-cis isomer under mildly acidic conditions. The N substituent of the carbamate was found to have a significant effect on the reaction, with an N-acetyl group facilitating rapid and complete α-anomerization. The orientation of the group close to the anomeric position was found to significantly contribute to directing the anomerization reaction.
By exploiting these findings, oligoaminoglycosides with multiple 1,2-cis glycosidic bonds were generated from 1,2-trans glycosides in a one-step process.
- Significant Substituent Effect on the Anomerization of Pyranosides: Mechanism of Anomerization and Synthesis of a 1,2-cis Glucosamine Oligomer from the 1,2-trans Anomer,
Shino Manabe, Hiroko Satoh, Jürg Hutter, Hans Peter Lüthi, Teodoro Laino, Yukishige Ito,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201303474