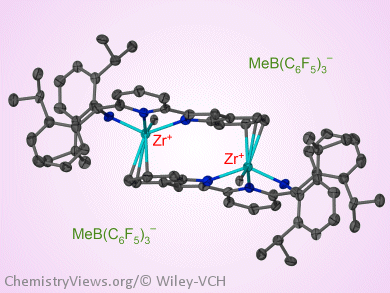
Metal-catalyzed olefin polymerization is a much investigated chemical reaction. To shed more light on the structure of the active species involved in this reaction, Claudio Pellecchia, Università di Salerno, and Alceo Macchioni, Università di Perugia, both Italy, and their groups have performed NMR spectroscopic and X-ray crystallographic measurements on dialkyl zirconium complexes based on tridentate dianionic N-heteroaryl-pyridylamido ligands: [(N–,N,N–)ZrR2]. After activation with AlMe3-depleted methylaluminoxane (DMAO)/AliBu2H, these complexes turned out to be highly active polymerization catalysts and gave ultra-high-molecular-weight polyethylenes and highly isotactic polypropylenes.
Their mechanistic studies have shown that the activation of [(N–,N,N–)ZrR2] dialkyl complexes with typical Lewis or Brønsted acid co-catalysts results, both in solution and in the solid state, in the unexpected formation of dimeric cations in which the pyrrolyl or indolyl moiety of one unit coordinates to the metal center of the other unit, whereas the counterions are pushed out of the first coordination sphere. It is thought that the formation of the dimeric species hampers monomer coordination and that addition of AliBu2H is crucial to split the homodimers.
Further studies are in progress to support these hypotheses and try to define the exact mechanism of activation of pyridylamido catalysts
- NMR Spectroscopy and X-ray Characterisation of Cationic N-Heteroaryl-Pyridylamido ZrIV Complexes: A Further Level of Complexity for the Elusive Active Species of Pyridylamido Olefin Polymerisation Catalysts,
G. Li, C. Zuccaccia, C. Tedesco, I. D’Auria, A. Macchioni, C. Pellecchia,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201303021