The use of graphene-based materials as catalysts and photocatalysts is provoking great interest owing to their potential as metal-oxide solids and TiO2 replacements. Graphene is a versatile material that can be modified by different strategies to improve its activity and thus provide greater sustainability and flexibility in the design of the photocatalysts. In addition, the use of carbon-based materials, such as graphene, is considered sustainable because they can be derived from renewable biomass feedstocks. In contrast, there is a limited resource availability of TiO2 and other metal-oxide semiconductors. Doping of TiO2 has also proven to be difficult, requiring harsh experimental conditions. In contrast, graphene-based materials can be modified by covalent or non-covalent attachment of photoresponsive units or even by replacement of some carbon atoms in the graphene layers by other heteroatoms.
Hermenegildo Garcia and co-workers, Universidad Politecnica de Valencia, Spain, have developed an N-doped graphene-based material by pyrolysis under an inert atmosphere of natural chitosan, which is considered a biomass waste, followed by ultrasound exfoliation.
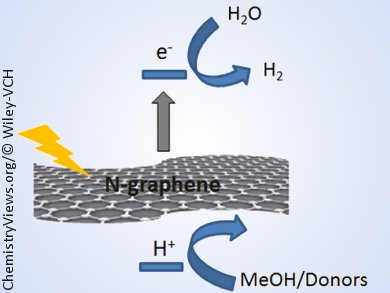
This material behaved as a semiconductor, exhibiting a high efficiency for the photocatalytic generation of hydrogen from water/methanol mixtures in the absence of Pt or any other metal. The efficiency of the material depended on the conditions of preparation, with the main parameter being the pyrolysis temperature, for which an optimal material was produced when the thermal treatment was carried out at 900 °C. The material exhibited photocatalytic activity upon UV (355 nm) and visible-light (532 nm) irradiation, thus generating hydrogen upon simulated sunlight illumination.
This work paves the way for the development of photocarbocatalysts that can exhibit photocatalytic activity without containing any precious or transition metals.
- N-Doped Graphene Derived from Biomass as a Visible-Light Photocatalyst for Hydrogen Generation from Water/Methanol Mixtures,
Cristina Lavorato, Ana Primo, Raffaele Molinari, Hermenegildo Garcia,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201303689