Metal complexes, including those of Pt, Au, As, and Sn, are currently used for the treatment of various medical conditions. However, all these examples are purely coordinative metal complexes. To expand the available chemical space is a current challenge in drug discovery. To this end, the anticancer activity of organometallic ruthenium compounds has been investigated.
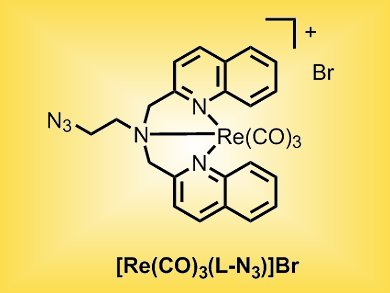
A team from Germany and Switzerland that includes Andreas Steffen, Nils Metzler-Nolte, Stefan Wölfl, and Gilles Gasser focusses on rhenium organometallic complexes. They have examined the photophysical and cytotoxic properties of the ReI complex [Re(CO)3(L-N3)]Br (L-N3 = 2-azido-N,N-bis[(quinolin-2-yl)methyl]ethanamine) and found that, besides the expected phosphorescence, the complex displays concentration-dependent residual fluorescence, which could explain why the complex’s fluorescence is quenched in live cells.
In addition, the complex shows cytotoxic activity in the low micromolar range in various cancer cell lines including MCF-7 breast cancer, U2OS osteosarcoma, and HepG2 liver cancer cells. [Re(CO)3(L-N3)]Br was found to be stable in human plasma. This indicates that [Re(CO)3(L-N3)]Br itself and not a decomposition product is responsible for the observed cytotoxicity. Further analysis revealed that the complex directly targets mitochondrial respiratory activity through two modes of action, namely increased respiration at lower concentrations, potentially by increasing proton transport through the inner mitochondrial membrane, and efficient blocking of respiration at higher concentrations.
These results make organometallic rhenium compounds a promising candidate for an anticancer drug.
- A Deadly Organometallic Luminescent Probe: Anticancer Activity of a ReI Bisquinoline Complex,
Igor Kitanovic, Suzan Can, Hamed Alborzinia, Ana Kitanovic, Vanessa Pierroz, Anna Leonidova, Antonio Pinto, Bernhard Spingler, Stefano Ferrari, Roberto Molteni, Andreas Steffen, Nils Metzler-Nolte, Stefan Wölfl, Gilles Gasser,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201304012